
Past The Cover Story


Forkhead box a2 (Foxa2) has been reported to play an important role in uterine function. However, the regulation of Foxa2 expression in the uterus during early pregnancy is still poorly understood. Yamagami et al. investigated the expression and regulation of Foxa2 in the rat uterus in the event of implantation (Yamagami et al. Expression and regulation of Foxa2 in the rat uterus during early pregnancy. pp. 468–475). It was observed that the expression of Foxa2 mRNA was transiently increased at 3.5 days post coitus and downregulated by 17β-estradiol. FOXA2 protein was localized especially in the nucleus of the glandular epithelial cells. Treatment of the epithelial cells with the recombinant Hedgehog protein significantly increased the expression of Foxa2 in vitro. In a nutshell, the expression profile and further localization of Foxa2 suggested that it could play an important role just before implantation and that it may be regulated by E2 and Hedgehog protein.

Histone modifications have important roles for biological processes. Therefore, researchers have investigated multiple cells and tissues. However, little is known about histone modification states during spermatogenesis. Shirakata et al. used immunohistochemical techniques to analyze the histone H4 modification pattern during murine spermatogenesis (Shirakata et al. Histone H4 Modification During Mouse Spermatogenesis. pp. 383–387). All the histone H4 modifications were high during the meiotic prophase, and histone H4 acetylation increased around step 10 spermatids. These results provide further insight into the specific relationship between histone H4 modifications and gene expression.


The CRISPR/Cas9 system is expected to be a powerful research tool for studies of genome editing in mammals. Fujii et al. verified the efficiency of the CRISPR/Cas9 system in generating triple-knockout mice (Fujii W, et al. One-step Generation of Phenotype-expressing Triple-knockout Mice with Heritable Mutated Alleles by the CRISPR/Cas9 System. pp. 324–327). A substantial proportion of the pups obtained carried loss-of-function mutations in all of the target alleles, exhibiting typical knockout phenotypes or no protein expression from the target genes. The induced mutations were inherited in the next generation, and therefore, the CRISPR/Cas9 system was demonstrated to be an efficient strategy for generating multiple gene knockout animals.


Zygote-specific proteasome chaperone (ZPAC), which is specifically expressed in the mouse gonad and zygote, mediates a unique proteasome assembly pathway in the zygote, but the expression profile and function of ZPAC in the testis are not fully understood. Shimizu et al. investigated the possible role of ZPAC during mouse spermatogenesis (Shimizu et al. Possible Role of ZPAC, Zygote-specific Proteasome Assembly Chaperone, during Spermatogenesis in the Mouse, pp. 179–186). In elongating spermatids, ZPAC was expressed until step 10. In addition, intense ZPAC staining was co-localized with staining of annexin V, an early indicator of apoptosis in mammalian cells, in germ cells of the cryptorchid testis, but ZPAC was also expressed in germ cells showing no detectable expression of annexin V. These results suggest that ZPAC plays a role during spermatogenesis.


In many seasonal breeding males, inactive spermatogenesis along with low testosterone concentrations has been detected in the non-mating season. However, in raccoons (Procyon lotor), which are seasonal breeders with a mating season in the winter, some males produce spermatozoa actively even in the summer non-mating season. Okuyama et al. reported that 3β-hydroxysteroid dehydrogenase expression may play an important role in regulating the seasonality of testosterone production (Okuyama et al. Changes in the Immunolocalization of Steroidogenic Enzymes and the Androgen Receptor in Raccoon (Procyon lotor) Testes in Association with the Seasons and Spermatogenesis, pp. 155–161). Immunohistochemical observation of testicular tissues, which exhibited active spermatogenesis in the summer, suggested that spermatogenesis in the raccoon testis might be maintained by some mechanism that regulates aromatase cytochrome P450 expression in the synthesis of estradiol and androgen receptor expression in the control of reactivity to testosterone.


Recent studies suggest that spermatogonial stem cells (SSCs) are not comprised of a pure population of cells, but the mechanism that underlies SSC heterogeneity has remained unknown. Ishii et al. investigated the contribution of cell cycle status to cell phenotype and SSC activity using fluorescent ubiquitination-based cell cycle indicator transgenic mice (Ishii K, et al. Cell-cycle-dependent Colonization of Mouse Spermatogonial Stem Cells After Transplantation into Seminiferous Tubules. pp. 37–48). Cultured spermatogonia enriched for SSCs in the G1 phase are significantly more efficient than those enriched in the S / G2-M phase in recolonizing the seminiferous tubules of adult mice following microinjection. These results suggested that cell cycle status is an important factor in regulating SSC activity.


Mitochondria are reported to be critical in in vitro maturation of oocytes and subsequent embryo development after fertilization, but their contribution to fertilization has not been investigated in detail. Viet Linh et al. investigated the contribution of mitochondria to fertilization using reconstructed porcine oocytes fused with ooplasmic fragments (Viet Linh N, et al. Fertilization Ability of Porcine Oocytes Reconstructed from Ooplasmic Fragments Produced and Characterized after Serial Centrifugations. pp. 549–556). Oocytes fused with mitochondria-rich fragments showed a higher incidence of sperm penetration and male pronuclear formation after in vitro fertilization compared with other types of reconstructed oocytes. These results suggested possible contribution of active mitochondria to fertilizability of oocytes.


Cohesin is a multi-subunit protein complex that plays an essential role in sister chromatid cohesion in mitosis and meiosis. Lee discussed the role of a new cohesin subunit, RAD21L (Lee: Roles of Cohesin and Condensin in Chromosome Dynamics During Mammalian Meiosis. pp. 431–436). In contrast to another meiosis-specific cohesin subunit that is expressed throughout meiosis, REC8, expression of RAD21L is restricted to prophase I. In mouse spermatocytes, RAD21L and its mitotic counterpart RAD21 are expressed in a mutually exclusive manner during meiosis: RAD21L is localized along the synaptonemal complex until mid pachytene, whereas RAD21 is localized there from then on. Later studies using knockout mice have demonstrated that RAD21L is involved in the formation of the synaptonemal complex as well as the synapsis and recombination of homologous chromosomes. However, the precise function of each cohesin subunit and the mechanism regulating their spatiotemporal expression remain to be elucidated.


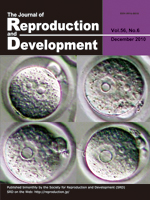
Hiraga et al. reported that the localization patterns of lipid droplets in the cytoplasm of porcine oocytes were evaluated as a novel marker for in vitro maturation of oocytes with high developmental competence (Hiraga et al.: Selection of In Vitro-Matured Porcine Oocytes Based on Localization Patterns of Lipid Droplets to Evaluate Developmental Competence. p. 405–408). Porcine oocytes were differentially stained with Nile red (red) and Hoechst 33342 (blue) for visualization of lipid droplets and nuclei, respectively. Localization patterns were divided into 2 classes: lipid droplets localized uniformly in the whole cytoplasm (class I) and those that were centrally located (class II). Class II oocytes showed a significantly higher rate of blastocyst development than class I oocytes

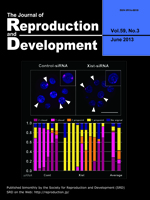
Ectopic expression of Xist is one of the major causes of inefficient somatic cell nuclear transfer in mice. It is known that prior injection of Xist-specific siRNA could significantly improve the birth rates of male clones. In this issue, Oikawa et al. report application of the same knockdown strategy to female clones (Oikawa et al.: RNAi-mediated Knockdown of Xist Does Not Rescue the Impaired Development of Female Cloned Mouse Embryos. pp. 231–237). RNA-FISH analysis revealed that siRNA treatment successfully repressed Xist RNA at the morula stage in female clones. However, unlike male clones, there was no significant improvement in the birth rates of the siRNA-injected embryos.


Campbell et al. discussed the use of insulin in embryo culture medium to improve murine embryo development, epiblast cell number, and the generation of embryonic stem cell (ESC) colonies (Campbell et al.: Epiblast cell number and primary embryonic stem cell colony generation are increased by culture of cleavage stage embryos in insulin. pp. 131–138). Embryonic outgrowths from early blastocysts and day 5 blastocysts had fewer epiblast cells, generated outgrowths at a lower rate, and were less likely to contain an epiblast than blastocysts plated on day 6. When day 6 blastocysts were cultured in insulin these factors were further improved. This demonstrated that culture of embryos in insulin only improves the quality of embryonic outgrowths when embryos are cultured to day 6 and have lineage commitment prior to plating. These results provide evidence for the usefulness of culturing embryos with insulin for increasing the yield of outgrowths with the potential to give rise to an ESC colony.


Bai et al. discussed the currently ascribed functions of the GATA family in trophoblasts during the peri-implantation period (Bai et al.: Expression and potential role of GATA factors in trophoblast development. pp. 1–6). In rodents, implantation occurs soon after blastocyst hatching from the zona pellucida, followed by several dramatic events toward placentation within a few days. In contrast, development of the post-hatching conceptus in ruminants uniquely features a prolonged peri-implantation period involving trophoblast elongation. Although the duration of peri-attachment periods and types of implantation vary, processes leading to conceptus implantation into the maternal endometrium including roles of GATA factors are similar in most mammalian species.

Matsumoto et al. report the mRNA distribution of the motin family in the mouse uterus (Matsumoto et al.: Differential expression of the motin family in the peri-implantation mouse uterus and their hormonal regulation. pp. 649–653). Motins are differentially expressed in uterine cells during the peri-implantation period. The postimplantation period from days 5 to 8 of gestation is characterized by expression of Amot and Amotl1 in secondary decidual cells and loss of Amotl2 expression. Steroid hormones such as P4 and E2 regulate differential expression of the motin family in the mouse uterus in a spatiotemporal manner.

Cai et al. report a lower than normal population of spermatogenic cells in the seminiferous tubules as well as a decreased number of spermatozoa in the lumen of the epididymides in HSV1-TK transgenic rats (Cai et al.: Accumulated HSV1-TK proteins interfere with spermatogenesis through a disruption of the integrity of Sertoli-germ cell junctions, pp. 544–551). In the testicular tissue of these transgenic rats that ectopically express the HSV1-TK gene by an inherent promoter, accumulation of HSV1-TK proteins causes a disorder in spermatogenesis. Microarray and real-time PCR analyses for testis RNAs suggest disruption of the integrity of Sertoli-germ cell junctions.

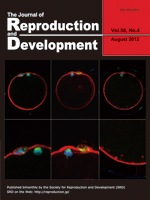
Naruse et al. report a treatment that elevates the cAMP levels of bovine in vitro matured oocytes, thereby improves the oocytes’ developmental competence and enucleation efficiency (Naruse et al.: Milrinone treatment of bovine oocytes during in vitro maturation benefits production of nuclear transfer embryos by improving enucleation rate and developmental competence, pp. 476–483). The improved enucleation rate is associated with the proximity of the metaphase plate location to the first polar body, indicating that normality of the cytoskeletal organization is crucial for successful enucleation. Adequacy of the treated oocytes as the recipient oocytes for nuclear transfer is indicated by the confocal images of the metaphase II spindle and cortical microfilaments, actin and tublin filaments, and chromosomes.


Fulka et al. report production of giant nucleoli obtained by enucleolation of mouse oocytes and their subsequent fusion (Fulka et al.: Production of giant mouse oocyte nucleoli and assessment of their protein content. pp. 371–376). The oocyte nucleoli (termed nucleolus precursor bodies) serve as the basis of active embryonic nucleoli; however, their composition is currently unknown. The enucleolation procedure in combination with colloidal gold protein labeling allows estimation of the average protein content of a single oocyte nucleolus. This information is crucial when levels of individual nucleolar proteins are to be compared between somatic cells and oocyte/embryonic nucleoli.

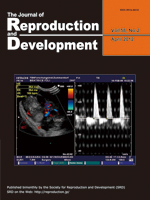
Brüssow et al. report Doppler images and spectral mode of the umbilical cord of a porcine fetus at day 36 of pregnancy using a laparoscopic guided ultrasound probe (Brüssow et al.: Laparoscopy guided Doppler ultrasound measurement of fetal blood flow indices during early to mid-gestation in pigs, pp. 243–247). This approach allows recording of blood flow characteristics of almost all fetuses in the crowded porcine uterus. Such recordings are not feasible by transcutaneous or transrectal ultrasound measurement. Based on the recordings, fetal size and heart rate parameters and flow indices as well as indexes of vascular resistance can be obtained. These measurements are applicable to interpretation of fetal development and blood supply, and intrauterine growth retardation (IUGR) in piglets.

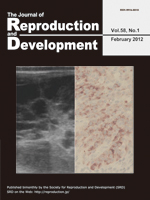
Granulosa-theca cell tumors (GTCTs) are the most common ovarian tumors in cattle. Kitahara et al. report, for the first time, the blood profile and tissue expression of anti-Müllerian hormone (AMH) in bovine GTCTs in this issue (Kitahara et al.: Anti-Müllerian hormone profiles as a novel biomarker to diagnose granulosa-theca cell tumors in cattle. p. 98–104). In the present cases of GTCTs, the ultrasound images of ovaries showed three different appearances of tumors, i.e., cystic, solid or both. Neoplastic granulosa cells were histologically labeled with AMH antibody. Blood AMH levels were significantly higher in cattle with GTCTs than in normal animals with cystic ovarian disease or superovulation treatment. The blood AMH level, therefore, may be a more useful biomarker to diagnose GTCTs than the traditional diagnostic aids. The JRD Editorial Board expresses its heartfelt condolences for the sudden death of Prof. Shunichi Kamimura, who was a coauthor of the present article and contributed to the journal as an editor for many years.


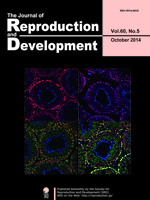
The 3D reconstruction analysis using confocal microscopy reveals functional interconnections among nonhypophysiotropic terminal nerve (TN)-GnRH3 neurons controlling motivation for reproductive behavior (Review article by Abe and Oka in this issue: Mechanisms of Neuromodulation by a Nonhypophysiotropic GnRH System Controlling Motivation of Reproductive Behavior in the Teleost Brain. p.665–674). Magenta shows immunofluorescence for anti-salmon GnRH (sGnRH), and green shows fluorescence for intracellularly injected biocytin. Approximately 10–15 TN-GnRH3 neurons form a cell cluster on both sides of the brain. It is noteworthy that fine somatic processes and varicose fibers containing GnRH peptides surround the somata (white). This confocal photomicrograph demonstrates the morphological basis for functional synchronization among TN-GnRH3 neurons by auto / paracrine release of GnRH.


Mercury light irradiation is a useful tool for observing fluorescence. An original article appearing in this issue (Terashita Y, et al.: Effect of fluorescent mercury light irradiation on in vitro and in vivo development of mouse oocytes after parthenogenetic activation or sperm microinjection. p. 564-571) reports that oocytes would be seriously damaged by irradiation with a short wave-length light for even short period. The cover of the present issues hows immunolocalization of Oct3/4 and Cdx2 in the blastocysts derived from oocytes irradiated with mercury light following ICSI. The quality of blastocysts irradiated with 341 nm light for 5 sec (at the bottom) is poorer than that of blastocysts irradiated with 341 nm light for 1 sec (at the top) in terms of cell number and Oct3/4 and Cdx2 expression pattern.


Arai et al. (Epigenetic Assessment of Environmental Chemicals Detected in Maternal Peripheral and Cord Blood Samples, p. 507–517) introduces a simple and sensitive method to detect het-erochromatin changes together with DNA methylation analysis to identify epimutagenic chemicals (epimutagens) that may affect early embryonic development. Exposure of mouse embryonic stem (ES) cells to certain chemicals, such as Se, as shown in the cover picture, was revealed to cause epigenetic changes (upper and lower panels: negative control and Se exposure, respectively), which were visualized by DNA-FISH with a specific probe for a heterochromatin marker, major satellite (red), merged with heterochromatin dot signals by DAPI staining (blue). Most importantly, their study has, for the first time, proven five chemicals(DEP, Hg, cotinine, Se and S-421) to be epimutagens even at the low concentrations that were detected in maternal cord blood samples.


Bovine oocytes were differentially immunostained by anti-tubulin (green) and DAPI (blue) for visualizing the microtubule network assembly and the position of nuclear materials, respectively. The proportion of oocytes that were injected with freeze-dried (FD) sperm and formed a sperm-aster was slightly lower than that of control ICSI oocytes. Among the oocytes exhibiting sperm-aster formation, the extent of microtubule net-work assembly was comparable between the FD-ICSI and control ICSI groups. However, the microtubule-organizing center of the ICSI oocytes was not as functional as that of IVF oocytes in terms of the aster formation rate and the fluorescent intensity of the microtubule network. (Hara H, et al.: Procedure for bovine ICSI, not sperm freeze-drying, impairs the function of the micro-tubule-organizing center. p.428–432)


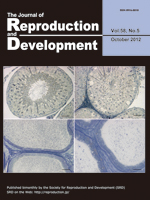
The review article by Inoue et al. in this issue (Inoue N, et al.: Role of cell-death ligand-receptor system of granulosa cells in selective follicular atresia in the porcine ovary. p. 169–175) describes that the apoptotic cascade via the death ligand/receptor system is activated in the granulosa cell during follicular atresia in the porcine ovary. Acivated caspase-3 (green) was found in granulosa cells with fragmented nuclei (red, PI staining) but not in cumulus cells in porcine progressed atretic follicles. Caspase-3 is an effector caspase that induces apoptotic cell death.


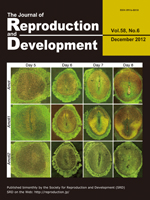
The vitrified-warmed bovine secondary follicle grew in size in a bovine plasma-supplemented medium. No distinct cavities were found in the follicle after 1 week of culture (top/left), but small cavities appeared after 2 weeks (top/right), which developed progressively larger after 3 weeks (bottom/left) and 4 weeks (bottom/right), eventually forming an antrum-like structure. The combination of cryopreservation and in vitro culture of growing ovarian follicles may be a promising method of providing a large number of mature oocytes. (Taketsuru, et al.: Bovine oocytes in secondary follicles grow in medium containing bovine plasma after vitrification. p.99–106).

Compared to untreated embryo (top/left), nuclei with a concave-like shape was observed in the pronuclei of 1-cell embryos transiently treated with MG132 in the G1 phase of the first cell cycle (top/right, and bottom/right and left), which may be similar to the unique structures termed as nucleolar caps. Interestingly, formation of nucleolar caps is known to be a normal cellular process associated with transcriptional shut down and drug-induced transcriptional arrest. The ubiquitin-proteasome system may be involved in transcriptional regulation in early mouse embryos. (Shin SW, et al.: Inhibition of the ubiquitin-proteasomes ystem leads to delay of the onset of ZGA gene expression. p. 655-663).


Most centromeres were localized around the nucleoli in the zygote (CREST in green). In addition, oocyte nucleolus loss alters the localization and size of centromeres in both male and female pronuclei, indicating that the nucleolus in the mouse oocyte organizes both paternal and maternal centromeric heterochromatin in pronuclei. The chromatin was stained positive for trimethylated histone H3 at lysine 9 (in red) in female pronuclei and negative in the male pronuclei. (Ogushi S and Saitou M: The Nucleolusin the Mouse Oocyte is Required for the Early Step of Both Female and Male Pronucleus Organization. p. 495–501)

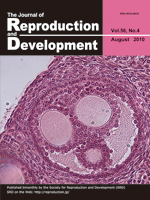
In the high fecundity mouse line FL1 of the Leibniz Institute for Farm Animal Biology (FBN, Dummerstorf), the number of ova ovulated was higher than the number of corpora lutea (CL) present. This observation suggests that individual follicles release more than one oocyte. Twenty-seven polyovular follicles per ovary were observed in this mouse line with some follicles containing up to 7 oocytes per follicle. The example shows a polyovular antral follicle with 3 oocytes in a mouse line selected for high fecundity. (Alm H, et al.: Occurrence of polyovular follicles in mouse lines selected for high fecundity. p. 449–453)

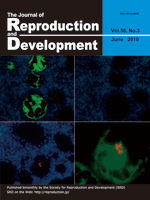
The localization and expression of chicken germline-specific proteins cDAZL, CDH and CVH are distinct in germ cells during the latter stage of gametogenesis. CDH (red) is found in the nucleus throughout oogenesis. CVH (green) is found in the nucleus and cytoplasmic compartments, although some oocytes are CVH-negative or have weak staining. However, cDAZL is not found in all oocytes (top/right), although cells positive for cDA-ZL and CVH coexist in the embryonic gonads. The lack of cDAZL may imply the initiation of meiosis. Blue: nuclear staining. (Kito G, et al.: Temporal and spatial differential expressionof chicken germline-specific proteins cDAZL, CDH and CVH during gametogenesis. p. 341–346)

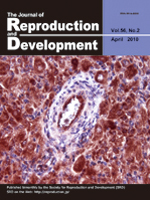
Immunolocalization of cellular FLICE-like inhibitory protein (cFLIP) in the bovine corpus luteum (CL) at the mid luteal stage. Hojo et al. (in this issue, pp. 230–235) show intense cFLIP immunoreactivities in luteal cells and endothelial cells that remain high during the early, developing and mid luteal stages and low in the late and regressed luteal stages. In addition, exposure of luteal cells to inflammatory cytokines decreased cFLIP expression and increased the number of apoptotic cells. They conclude that cFLIP plays a survival role in the bovine CL.
