
CoverStory


In mammalian female reproduction, primordial follicles serve as stores to sustain the ovulation cycle. Regulations of primordial follicle development, activation, and dormancy in mice are summarized in a review by Nagamatsu (p. 189–195). The importance of mechanical stress, especially extracellular matrix (ECM)-mediated pressure, for the maintenance of primordial follicle dormancy was recently demonstrated. Primordial follicles treated with collagenase, trypsin, and knockout serum replacement (KSR) (this mixture called CTK) to digest ECM were examined by immunohistochemistry. Ovaries treated solely with phosphate-buffered saline displayed primordial follicles composed of flat granulosa cells and complex stress fibers, as revealed by phalloidin. In contrast, CTK treatment resulted in fewer stress fibers and cuboidal-shaped granulosa cells, suggesting oocyte activation.

Recent studies suggested that a small sub-population of embryonic stem (ES) cells exhibit 2-cell stage embryo-like (2-cell-like) features, including the reactivation of murine endogenous retrovirus with leucin transfer RNA primer, high histone mobility, and dispersed chromocenters. Furuta et al. investigated the organelle morphology of 2-cell-like cells using electron microscopy (Furuta et al. Lipid droplets are formed in 2-cell-like cells. pp. 79–81). They demonstrated the formation of a lipid droplet during the transition from ES cells to 2-cell-like cells, and proposed that these cells utilize a unique energy storage and production pathway.

Previous reports suggested the involvement of alpha-tubulin N-acetyltransferase 1 (ATAT1) in flagella formation in spermatozoa; however, whether ATAT1 is expressed in flagella and involved in spermatozoa maturation and capacitation remains to be elucidated. Yanai et al. evaluated the expression of ATAT1 in the male reproductive system in mice using immunostaining and western blotting (Yanai et al. Expression and localization of alpha-tubulin N-acetyltransferase 1 in the reproductive system of male mice. pp. 59–66). The localization of ATAT1 protein in the male germline was detected during spermiogenesis as well as spermatozoa maturation. Therefore, these results suggest that ATAT1 might be involved not only in flagella formation, but also in the acetylation process during spermatozoa maturation and capacitation

Cluster of differentiation 9 (CD9) forms a complex with CD81, and is a member of the tetraspanin superfamily. Cd9 and Cd81 are highly expressed in breast cancer cells, but their expression in healthy mammary glands remains unclear. Horiguchi et al. reported expression of Cd9 and Cd81 in mammary epithelial cells, with expression levels correlating with mammary gland development (Horiguchi et al. Expression and functions of cluster of differentiation 9 and 81 in rat mammary epithelial cells, pp. 515–522). To examine the functional roles of CD9 and CD81, Horiguchi et al. knocked down Cd9 and Cd81 gene expression using small interfering RNAs (siRNAs) in isolated CD9-positive mammary epithelial cells. They found that siRNAs against Cd9 and Cd81 inhibited estrogen-induced mammary epithelial cell proliferation. These findings provide novel insights into mammary epithelial cell proliferation during pregnancy and lactation, as well as in pathological processes associated with breast cancer.

Loss of genomic integrity in preimplantation embryos can cause not only developmental arrest but also diseases such as congenital disorders and cancers. However, the key factors for maintaining genomic integrity in preimplantation embryos remain unknown. Shikata et al. reported that SETD8- mediated monomethylation of H4K20, a type of epigenetic modification, plays an important role in the maintenance of genomic integrity (Shikata et al. H4K20 monomethylation inhibition causes loss of genomic integrity in mouse preimplantation embryos. pp. 411–419). In our study, the inhibition of SETD8 or the overexpression of dominant-negative histone H4 mutants resulted in developmental arrest and excessive accumulation of DNA double-strand breaks, suggesting that H4K20 monomethylation is associated with DNA damage repair and is essential for preimplantation development.


Spermatogonial stem cells (SSCs) comprise merely a small population in the testis. To facilitate studies on SSCs, it is necessary to find markers that are expressed thereon. Kanatsu-Shinohara et al. discovered a new surface marker on mouse and rat SSCs known as cluster of differentiation 2 (CD2) while studying the molecular mechanism of SSC aging (Kanatsu-Shinohara et al. CD2 is a surface marker for mouse and rat spermatogonial stem cells. pp. 341–349). Using a fluorescence-activated cell sorter, SSCs were enriched by 291.9-fold compared to those in wild-type mouse testes, based on CD2 expression. CD2 depletion by short hairpin RNA in cultured SSCs compromised their stem cell activity. Conserved CD2 expression on mouse and rat SSCs suggest that CD2 may also be expressed on SSCs from other animal species, including humans.

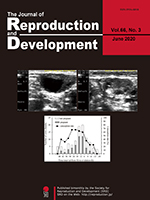
To improve the reproductivity of dairy cows, which has been continuously decreasing, it is important to note that the detection of estrus should be accurate and that the timing of artificial insemination (AI) should be appropriately decided. However, the relationship between the conception rate and the timing of AI after the onset of estrus or ovulation period, following the performance of AI using the frozen-thawed semen, has not been investigated yet. However, Sumiyoshi et al. (2020) investigated the relationship between the ovulation time after AI and the conception rate, and identified the time span during which the frozenthawed semen delivers high conception rate after its deposition in the uterus (Sumiyoshi et al., An investigation of the time period within which frozen-thawed semen delivers a high conception rate in lactating dairy cows, pp. 277–280). In this study, the authors demonstrated that high conception rates averaging 63.0% were obtained by performing AI 6–30 h before ovulation, which was significantly higher than the conception rates of 30.0% (P < 0.05) and 26.9% (P < 0.01) obtained from AI that was performed earlier than 30 h before ovulation and later than 6 h before ovulation, respectively. These results further suggest that the frozen-thawed semen can deliver a conception rate of ≥ 60% if it deposits in the uterus for more than 24–30 h after AI, and also that a conception rate of ≥ 60% can be achieved by performing AI 6–30 h before ovulation by using the frozen-thawed semen.


Most primordial follicles present in ovaries are dormant and only a few of them are activated in every estrus cycle. However, the mechanism controlling the activation of dormant primordial follicles in vivo remains unclear. In this study, Komatsu et al. found that almost all the activated primordial follicles (black arrows) made contact with blood vessels (red arrows) in mouse ovaries (Komatsu et al. Increased supply from blood vessels promotes the activation of dormant primordial follicles in mouse ovaries. pp. 105–113). To confirm the hypothesis that angiogenesis is crucial for activation of the dormant primordial follicles in vivo, Komatsu et al. induced angiogenesis using recombinant VEGF. They found that the activation of dormant primordial follicles was promoted by an increase in the number of blood vessels in the ovaries. Furthermore, the number of activated follicles increased in cultured ovarian tissues depending on the serum concentration in the medium. These results confirm that the supply of serum components through new blood vessels formed via angiogenesis is a cue for the activation of dormant primordial follicles in the ovaries.

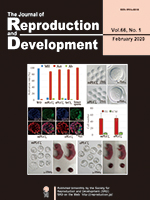
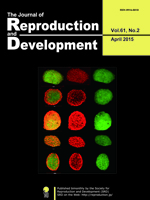
Improving artificial oocyte activation is essential for animal biotechnology, to obtain healthy offspring with a high success rate. Yamamoto et al. investigated whether the equine sperm-specific phospholipase C zeta (ePLCζ) mRNA, which has the strongest oocyte activation potential in mammals, could improve the mouse oocyte activation rate and subsequent embryonic development using inactivated spermatozoa (Yamamoto et al. Production of mouse offspring from inactivated spermatozoa using horse PLCζ mRNA. pp. 67–73). The activation potential of ePLCζ was ten times greater than that of murine (m) PLCζ and normal blastocysts were obtained. However, the birth rate was slightly, but significantly, decreased in oocytes activated by ePLCζ compared to those activated by mPLCζ. These results suggest that activation rate does not always correlate birth rate.


K Takeda. Functional consequences of mitochondrial mismatch in reconstituted embryos and offspring, pp. 485–489
Animal cloning technology has been developed to produce progeny genetically identical to a given donor cell. However, recipient oocytes contribute a heritable mitochondrial genomic (mtDNA) background to the progeny. In the somatic cell nuclear transfer (SCNT) process, a small amount of donor cellderived mitochondrial genome (D-mtDNA) can accompany the transferred nucleus; therefore, mtDNA from two sources is combined in the cytoplasm (heteroplasmy) of the reconstituted oocyte. The proportion of D-mtDNA can be restricted in animals produced by SCNT due to random distribution, selective replication/degradation associated with cell division during early embryo development, or maternal inheritance of germplasm. These phenomena are described as bottleneck effects (BN1-3). The mtDNA bottleneck characterized by rapid generational shifts in maternal germline can result in both mtDNA heteroplasmic and homoplasmic lines in subsequent generations.
Animal cloning technology has been developed to produce progeny genetically identical to a given donor cell. However, recipient oocytes contribute a heritable mitochondrial genomic (mtDNA) background to the progeny. In the somatic cell nuclear transfer (SCNT) process, a small amount of donor cellderived mitochondrial genome (D-mtDNA) can accompany the transferred nucleus; therefore, mtDNA from two sources is combined in the cytoplasm (heteroplasmy) of the reconstituted oocyte. The proportion of D-mtDNA can be restricted in animals produced by SCNT due to random distribution, selective replication/degradation associated with cell division during early embryo development, or maternal inheritance of germplasm. These phenomena are described as bottleneck effects (BN1-3). The mtDNA bottleneck characterized by rapid generational shifts in maternal germline can result in both mtDNA heteroplasmic and homoplasmic lines in subsequent generations.


The oocyte is the only cell that can reprogram a somatic nucleus to totipotency. The process of reprogramming is, however, only partially understood, and is accompanied by both epigenetic and structural changes in the somatic nucleus. The oocyte components that are necessary for a successful reprogramming and remodeling are unknown. In this issue, Fulka H et al. demonstrate that rather than the insoluble nuclear envelope, together with chromatin-bound factors, or the cytoplasm alone, it is the soluble nuclear fraction that has a major effect upon the somatic nucleus (Fulka H, et al.: Dissecting the role of the germinal vesicle nuclear envelope and soluble content in the process of somatic cell remodeling and reprogramming. pp. 433–441). This fraction is essential for altering the size of the somatic nucleus as well as transcriptional silencing and efficient histone H3.3 incorporation.


In vitro/ex vivo egg production has been widely studied in various mammalian species over half of the century to utilize the majority of the immature oocytes stocked in the female ovaries. Recently, the first successful protocol of in vitro oogenesis from primordial germ cells (PGC) has been established by Morohaku K, et al., resulting in the live birth of offspring in mice. The protocol consists of two vital steps; 1) ex vivo organ culture of mouse PGC ovaries to complete the process of follicle formation, with successful incorporation of antagonists for the existing estrogen receptors, and 2) in vitro follicle culture of the growing follicles isolated from the cultured ovaries. The review in this issue introduces the current findings and aspects governing in vitro oogenesis, with a brief history (Morohaku K. A way for in vitro/ex vivo egg production in mammals. pp. 281–287).


In cattle, small oocytes (group VII), included in small follicles, grow with follicular development, and the size and developmental competence of oocytes increase (groups I and II) (Nagano, Acquisition of developmental competence and in vitro growth culture of bovine oocytes, pp. 195–201). Subsequently, only one follicle is selected to develop to a dominant follicle and ovulates, but the other follicles start to degenerate. During the degeneration process, an accumulation of lipid droplets and undulation of the nuclear membrane of germinal vesicle start, and the developmental competence of oocytes also increases (pseudomaturation-like change in group III). However, too many pseudomaturation-like changes impair the developmental competence of oocytes (groups V and VI). If oocytes start to degenerate before pseudomaturation-like changes, the oocytes may become group IV.


The mitochondrial sheath is composed of mitochondria that coil tightly around the midpiece of the sperm flagellum. Mitochondria are recruited from the cytoplasm to the flagellum late in spermatogenesis. Recruited mitochondria are initially spherical, but then elongate laterally to become crescent-like in shape. Subsequently, these crescent-like mitochondria elongate continuously to coil tightly around the flagellum. Mitochondrial sheath development in glycerol kinase 2 (Gk2)-disrupted mice, which show abnormal mitochondrial sheath formation, was observed using freeze-fracturing coupled with scanning electron microscopy (Shimada et al., Glycerol kinase 2 is essential for proper arrangement of crescent-like mitochondria to form the mitochondrial sheath during mouse spermatogenesis, pp. 155–162). Gk2-disrupted spermatids show abnormal localization of crescent-like mitochondria, despite initially exhibiting proper alignment of spherical mitochondria around the flagellum. These results indicate that GK2 is essential for proper arrangement of crescent-like mitochondria during mitochondrial sheath formation in mouse spermatogenesis.


Mammalian oocyte quality degrades over time after in vitro ovulation. As various oocyte manipulations employed in assisted reproductive technology are time consuming, post-ovulatory aging is a serious problem in reproductive medicine and ova research. Shimoi et al. investigated the effects of post-ovulatory aging on the incidence of chromosomal aneuploidy during meiosis II (MII), with a focus on the expression of functional proteins from the spindle assembly checkpoint (SAC) (Shimoi G et al.: Destabilization of spindle assembly checkpoint causes aneuploidy during meiosis II in murine post-ovulatory aged oocytes. pp. 57–66). This study showed that post-ovulatory oocyte aging inhibits MAD2 localization to the sister kinetochore. Furthermore, oocyte aging prevented cohesin subunits from being appropriately maintained or degraded. These results suggest that destabilization of SAC signaling causes sister chromatid segregation errors in MII oocytes and consequently increases the incidence of aneuploidy in early embryos.


The review article by Uenoyama et al. describes the roles of hypothalamic kisspeptin in the central mechanism regulating puberty and subsequent reproductive functions in mammals. The schematic illustration shows a possible mechanism regulating the pubertal augmentation of Kiss1 expression in the arcuate nucleus (ARC) to trigger pulsatile GnRH/ gonadotropin secretion in rodents. The authors suspect that estrogen strongly suppresses ARC Kiss1 expression during the prepubertal period via direct and indirect pathways and that the sensitivity to estrogen negative feedback action on ARC Kiss1 expression decreases during the pubertal transition. The resultant increase in secretion of kisspeptin would trigger GnRH/gonadotropin secretion at pubertal onset (Uenoyama et al. The role of kisspeptin in the mechanism underlying reproductive function in mammals. pp. 469–476).


Dry biobanking has many advantages over the current paradigm of storing cryopreserved cells under liquid nitrogen. During drying, however, the cells become damaged. The highly condensed spermatozoa DNA has been shown in many desiccation studies to generally maintain its integrity. Using ram freeze-dried epididymal spermatozoa as a model, Palazzese et al. were the first to evaluate both single- and double-strand DNA breaks (SSBs and DSBs, respectively), showing that drying causes minimal DSBs but extensive SSBs (Palazzese L et al., 2018. DNA fragmentation in epididymal freeze-dried ram spermatozoa impairs embryo development, pp. 393–400). Furthermore, the authors also demonstrated that spermatozoa capable of directing embryo development to the blastocyst stage in vitro originated from rams with the least DNA damage Overall, the impact of sperm DNA damage on embryonic development depends on a balance between the extent of sperm DNA fragmentation, fragmentation type, and the oocyte’s repair capacity.


Oog1, an oocyte-specific gene, encodes the protein belonging to the leucine-rich repeat (LRR) superfamily. LRR is a motif involved in protein-protein interactions. Complete knockout of Oog1 is challenging because five copies of the Oog1 gene are present on chromosomes 4 and 12. Honda et al. generated Oog1 RNA interference (RNAi)-transgenic mice to investigate the effects of Oog1 knockdown on gene expression in the oocytes (Honda et al. Oocyte-specific gene Oog1 suppresses the expression of spermatogenesis-specific genes in oocytes, pp. 297–301). The abundance of spermatogenesis-specific transcripts was elevated in the Oog1 knockdown ovaries. In addition, a few abnormal oocytes were observed in 6-month-old Oog1 knockdown mouse ovaries. These findings suggested that OOG1 suppresses the expression of spermatogenesis-specific genes in the oocytes and plays important roles during oogenesis.


Based on the results obtained for the studies of in vitro development of cloned embryos, epigenetic modifications have been widely used for cloning farm animals. However, such studies remain few in canids because of the lack of optimal in vitro oocyte maturation, embryo culture, and superovulation system. Kim et al. investigated whether a histone deacetylase inhibitor used in dog to pig interspecies somatic cell nuclear transfer (iSCNT), which improves nuclear reprogramming, could be used in dog cloning (Kim et al.: Suberoylanilide hydroxamic acid during in vitro culture improves development of dog-pig interspecies cloned embryos but not dog cloned embryos. p. 277–282). Porcine oocytes supported reprogramming of nuclei from dog fibroblasts up to early developmental stage of iSCNT embryos, and treating the embryos with suberoylanilide hydroxamic acid (SAHA) increased their developmental competence. However, unfortunately, SAHA treatment for dog to pig iSCNT embryos was not sufficient to improve their in vivo development because three and one clones were successfully produced from the control and SAHA treated groups, respectively.

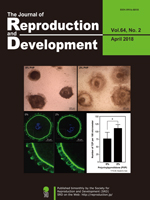
Recently, the effects of macromolecular crowding on cultured cells have gathered increasing interest. Crowded culture medium prepared by the addition of polyvinylpyrrolidone (PVP; Mw 360,000) positively influences the viability of oocytes during in vitro growth. Mizumachi et al. found that crowding affects a wide range of factors, including oocyte viability, complex morphology, and intimate conjunction of oocytes with cumulus/granulosa cells across the zona pellucida (Mizumachi et al. Macromolecular crowded conditions strengthen contacts between mouse oocytes and companion granulosa cells during in vitro growth, pp. 153–160). Confocal laser scanning microscopy indicated a higher number of transzonal processes (TZPs) reaching the oocyte from cumulus cells in 2% PVP medium than in 0% PVP medium.


Whether cumulus cells are required for the successful vitrification of mature oocytes in cattle is controversial. Ishii et al. re-evaluated the effects of the presence of multi-layered cumulus cells (MCCs) on the vitrification of mature bovine oocytes (Ishii et al., Embryogenesis of vitrified mature bovine oocytes is improved in the presence of multi-layered cumulus cells. pp. 95–99). In the presence of MCCs, there was no difference between the embryonic development of fresh and vitrified mature bovine oocytes. These results suggest that MCCs protect mature oocytes from freezing, and promote their survival and development after in vitro fertilization. Ishii et al. successfully produced calves using a small number of vitrified mature oocytes with MCCs collected from the ovaries of individual cows post-slaughter.

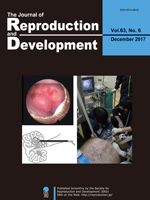
Okuyama et al. reported a transvaginal endoscopy-based technique for conducting ovarian examination in sows in a standing position. Sows were sedated in pig stalls, and their vaginal walls were punctured. Subsequently, a urethroscope was inserted into their abdomen, and an examination was conducted after their ovaries had moved towards the urethroscope camera via rectal palpation (Okuyama MW, et al. A transvaginal endoscopy-based technique for performing ovarian examinations in sows. pp. 617–622). This less invasive procedure without the use of general anesthesia may allow repeated ovarian examinations and increase our understanding of the ovarian dynamics in pigs.

Geminin is involved in the regulation of DNA replication and serves as a transcriptional molecular switch that directs cell fate decisions. Yuan et al. investigated the functions of geminin in postnatal, pre-meiotic male germ cells by using the Stra8-Cre/loxP system (Yuan et al. Geminin deletion in pre-meiotic DNA replication stage causes spermatogenesis defect and infertility, pp. 481–488). Geminin-deficient mice exhibited smaller testes compared to the control group, concomitant with few spermatozoa in the cauda epididymis and the severe loss of germ cells. These results suggest that geminin plays important roles in pre-meiotic DNA replication and subsequent spermatogenesis.

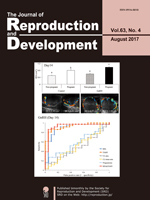
Gonadotropin-releasing hormone (GnRH), a neuropeptide released from the hypothalamus, has a central role in reproductive function in mammals; however, its role in luteal vascularity has not yet been analyzed. Kanazawa et al. investigated the effects of GnRH agonist treatment on Day 5 (Day 0 = estrus) on luteal blood flow and accuracy of pregnancy prediction in recipient cows (Kanazawa et al., Administration of gonadotropin-releasing hormone agonist on Day 5 increases luteal blood flow and improves pregnancy prediction accuracy on Day 14 in Holstein recipient cows, pp. 389–399). The maximum-diameter luteal blood flow areas in GnRH-treated cows were significantly higher than those in the non-treated cows on Days 7 and 14. In addition, the sensitivity and specificity of pregnancy prediction on Day 14 using the paired blood flow area and time-averaged maximum velocity at the spiral arteries were increased by the GnRH treatment. These results suggest that GnRH treatment on Day 5 increases the luteal blood flow on Days 7 and 14, and improves the accuracy of pregnancy prediction in recipient cows on Day 14.


Well-organized mitochondrial functions and dynamics are critical for early embryonic development, but their mechanisms are poorly understood. Ren et al. performed a high-resolution dynamic profiling of mitochondria-related genes (MtGs) to acquire a more detailed understanding of mitochondrial modifications during early development (Ren et al. High-resolution profiles of gene expression and DNA methylation highlight mitochondrial modifications during early embryonic development, pp. 247–261). Their results suggest that the resumption of mitochondrial mass growth is facilitated not only by increased mitochondrial biogenesis and mitochondrial DNA (mtDNA) replication, but also by the appropriate balance between mitochondrial fission and fusion (upper figure). In addition, increased levels of reactive oxygen species (ROS) resulting from enhanced mitochondrial function may be the critical inducer for activating the glutathione (GSH)-based stress response system in early embryos (lower figure).


During the process of zygotic gene activation (ZGA), which occurs in the fertilized oocyte, genomic function is regulated by dynamic structural changes in the nucleus. Nakaya et al. developed an easy-to-use chamber device (EASI-FISH chamber) for three-dimensional fluorescence in situ hybridization (3D-FISH) in the embryos and succeeded in visualizing the intra nuclear spatial arrangements in the fertilized eggs (Nakaya M, et al., Visualization of the spatial arrangement of nuclear organization using threedimensional fluorescence in situ hybridization in early mouse embryos: A new “EASI-FISH chamber glass” for mammalian embryos. pp. 167–174). Pericentromeric regions which comprise heterochromatin clusters called chromocenters in somatic cells decondensed during the 1- to 2-cell stages and changed to the chromocenter-like structure thereafter. In the early embryos, the ZGA-related Zscan4 gene loci were located peripherally in the CTs but relocated interiorly in somatic cells. In addition, the CTs in the early embryos were enlarged and had deformed shapes, which were quite different from those in somatic cells. This convenient and reproducible 3D-FISH technique for mammalian embryos is a valuable tool that will provide insights into the nuclear dynamics during development.

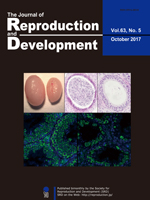
Cell-secreted vesicles, such as exosomes, have been recognized as mediators of cell communication. In recent studies, exosomes were shown to be involved in the regulation of ovarian function including in the induction of cumulus expansion. To date, exosome function has not been investigated in pigs. Matsuno et al. reported the existence of exosome-like vesicles in porcine follicular fluid and examined the effects of these vesicles on cumulus expansion in porcine cumulus-oocyte complexes (Matsuno et al., Effects of exosome-like vesicles on cumulus expansion in pigs in vitro, pp. 51–58). The existence of exosome-like vesicles was confirmed by transmission electron microscopic observation (top left: negative stain method image; top right: cryo-TEM method image). The vesicles labeled with fluorescent tags were incorporated into cumulus and mural granulosa cells in vitro (bottom left and right: vesicles shown in green with white arrowhead). The vesicles had no significant effects on the expansion of porcine cumulus-oocyte complexes in vitro. Therefore, although exosome-like vesicles exist in ovarian follicles, they are not efficient in inducing cumulus expansion in pigs in vitro.


Meiosis-specific α-kleisin subunits (RAD21L and REC8) play roles in the formation of axial elements, assembly of the synaptonemal complex, recombination of homologous chromosomes, and cohesion of sister chromatids. However, the exact functions of the individual α-kleisin subunits remain to be elucidated. Using 3D-SIM, Rong et al. found that RAD21L and REC8 were localized at the connection sites between lateral elements and transverse filaments of pachynema with RAD21L locating interior to the REC8 sites (Rong et al.: Meiotic cohesin subunits RAD21L and REC8 are positioned at distinct regions between lateral elements and transverse filaments in the synaptonemal complex of mouse spermatocytes. pp. 623−630). Intriguingly, some bridge-like signals of RAD21L, but not REC8, were observed between unsynapsed regions of axial elements of zygonema. Furthermore, the signals of recombination intermediates overlapped with those of RAD21L to a greater degree than with the signals of REC8. These results highlight the different properties of the two meiotic α-kleisin subunits, and support the view that RAD21L is an atypical cohesin that establishes the association between homologous chromosomes rather than sister chromatids.


Appeltant et al. reviewed the involvement of cAMP in maturation regulation, the function of oocyte-secreted factors (OSFs), and the interaction of both in the bidirectional regulatory loop between porcine oocytes and their cumulus cells. (Appeltant et al. Porcine oocyte maturation in vitro: role of cAMP and oocyte-secreted factors—A practical approach, pp. 439–449). In vivo, follicle-stimulating hormone induces luteinizing hormone (LH) receptors and epidermal growth factor (EGF) receptors (EGFR). LH can stimulate the LH receptor, while EGFR can be stimulated by amphiregulin, epiregulin, and betacellulin. In vitro, EGF cannot activate the EGFR and extracellular signal regulated kinase (ERK1/2) signaling in cumulus cells of porcine cumulus oocyte complexes derived from small antral follicles (< 4 mm), leading to a low developmental competence of these oocytes. The co-operative action of dibutyryl cAMP sodium salt and OSFs in an amphiregulin-stimulated in vitro maturation system promotes the EGFR/ERK1/2 signaling pathway, consequently improving the developmental potential of the oocytes.


The mechanism of bovine sperm functional regulation in vivo is still largely unknown. By using spermatozoa from elite bulls (top left), Umezu et al. have proposed a new regulation model of bovine sperm functions mediated by the neurotensin (NT) ligand-receptor system (Umezu et al.: Exogenous neurotensin modulates sperm function in Japanese Black cattle. pp. 409–414). They confirmed the expression of neurotensin receptor 1 (NTR1) in the bovine sperm neck region (top right) and the secretion of NT in the bovine uterus and oviduct. They also examined the effects of exogenous NT on bovine sperm functions such as hyperactivation (middle right). Exogenous NT enhanced protein tyrosine phosphorylation in bovine sperm and acrosomal disappearance rates in a dose-dependent manner in vitro (middle left). These results suggest that NT acts as a facilitator of sperm capacitation and acrosome reaction in the female reproductive tract in cattle, thus highlighting the importance of NT-mediated signaling to regulate sperm functions (bottom).


Data on pituitary transcription factors have demonstrated the regulatory mechanisms of the three pituitary glycoprotein hormone subunit genes (Fshβ. Lhβ, and Cga) constituting two types of gonadotropins. However, the functional differences between the LIM-homeobox transcription factors LHX2 and LHX3 in the regulation of Fshβ and Cga remain unclear. Yoshida et al. reported the DNA binding properties and transcriptional activities of Cga and Fshβ between LHX2 and LHX3 as well as their localization in the pituitary gonadotrope of rat (Yoshida et al., Transcription of Follicle-Stimulating Hormone Subunit Genes is Modulated By Porcine LIM Homeobox Transcription Factors, LHX2 and LHX3. pp. 241–248). In particular, immunohistochemical analysis demonstrated a rare population of LHX2 in contrast to LHX3 in the gonadotrope. The present study showed that LHX2 and LHX3 differentially regulate Fshβ and Cga.


Miyahara et al. revealed that the membrane-bound form of chicken stem cell factor (chSCF2) was highly beneficial for the in vitro culture of chicken primordial germ cells (PGCs) and that it exerted its growth-promoting effects by cooperating with fibroblast growth factor 2 (Miyahara et al., Chicken stem cell factor enhances primordial germ cell proliferation cooperatively with fibroblast growth factor 2, pp. 143–149). The cultured PGCs were maintained as large spheres (differential interference image) on a feeder layer stably expressing chSCF2. These cultured PGCs expressed the germ cell marker CVH (green) and the undifferentiated cell marker SSEA-1 (red). The cultured PGCs were isolated from a white donor chicken and then transplanted into a recipient embryo from a black chicken, generating a germline chimeric chicken expected to possess both donor- and recipient-derived sperm. The presence of a white chick among the offspring of this chimeric chicken demonstrates that these cultured PGCs were able to undergo normal gametogenesis. These results suggest that chSCF2 induces hyperproliferation of chicken PGCs while retaining their germline competency.

In vitro growth of immature oocytes provides the opportunity to increase gametic resources and to understand the mechanisms underlying oocyte development. Eppig et al. demonstrated that non-growing oocytes in primordial follicles can develop into functional oocytes in vitro; however, no one has been able to reproduce such excellent data in the last 20 years. Morohaku et al. established a novel and robust protocol for producing mature oocytes from non-growing oocytes in vitro (pp. 1–5). The addition of fetal bovine serum and polyvinylpyrrolidone to the medium is the primary difference between the protocols established by Morohaku et al. and that of Eppig et al. The resulting oocytes can develop into fertile offsprings after in vitro fertilization and embryo transfer. The birth rate, by this method, greatly exceeds that of the previous study. Although there is still scope for further improvement, Morohaku’s report will facilitate the in vitro growth of mammalian oocytes for practical applications.


Artificial insemination (AI)-subfertile bulls have occasionally been found in the sire candidates of high-performance Japanese Black cattle. Kishida and Sakase et al. reported that it is valid to perform exact examinations of acrosomal conditions of cryopreserved spermatozoa for exclusion of these subfertile bulls from the AI program for Japanese Black cattle (Kishida and Sakase et al. Effects of acrosomal conditions of frozen-thawed spermatozoa on the results of artificial insemination in Japanese Black cattle, pp. 519–524). The acrosomal conditions were assessed by peanut agglutinin-lectin staining and immunostaining of acrosomal tyrosine-phosphorylated proteins. The percentages of cryopreserved spermatozoa with normal acrosomal conditions were significantly correlated with the conception rates of routine AI, rates of transferable embryos in superovulation/AI-embryo collection tests and in vitro fertilization rates. These results indicate that low conception rates in AI using cryopreserved spermatozoa with poor acrosomal conditions result from reproductive dysfunctions in the processes between sperm insemination into females and early embryo development, probably from failed fertilization of oocytes with cryopreserved spermatozoa.

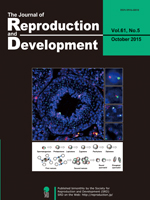
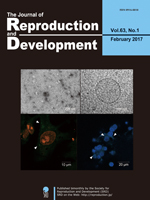
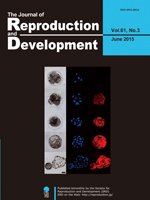
The dynamics of sex chromosomes during spermatogenesis have been unclear in vivo. Otaka et al. showed the localization of sex chromosomes before and after meiosis in mouse testis sections by fluorescence in situ hybridization (FISH) using specific probes for the X and Y chromosomes (Otaka et al. Distribution of the sex chromosome during mouse spermatogenesis in testis tissue sections. pp. 375–381). The positions of the X chromosomes (red) and Y chromosomes (green) were detected in spermatogenic cells (nucleus; blue), and the dynamics of sex chromosomes during spermatogenesis were demonstrated.

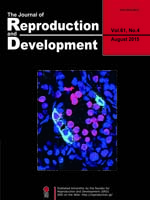
Although Rb1 is reportedly essential for self-renewal of spermatogonial stem cells (SSCs), its mechanism has remained unknown. Tanaka et al. examined the phenotype of Rb1-deficient SSCs in detail using cultured SSCs and conditional knock-out mice and found that Rb1 is critical for protection from genomic damage, which induces cell cycle arrest and apoptosis (Tanaka et al. The CDKN1B-RB1-E2F1 pathway protects mouse spermatogonial stem cells from genomic damage. pp.305–316). This picture shows increased expression of γH2AX (a double-strand break marker, red) in GFRA1(green)-expressing undifferentiated spermatogonia in 10-day-old Ddx4-Cre Rb1flox/– mice, in which Rb1 is specifically inactivated in germ cells (blue: Hoechst 33342).

Monomeric Plum, a far-red fluorescent protein with photostability and photopermeability, is a potentially suitable cell marker for a variety of animal experiments. Watanabe et al. created a transgenic cloned pig systemically expressing Plum (Watanabe et al. Production of transgenic cloned pigs expressing the far-red fluorescent protein monomeric Plum. pp. 169–177). Expression of Plum commenced at the early embryonic stage and was confirmed in all of the tissues/organs examined, including blood cells. Cells expressing Plum could be clearly distinguished in culture or by flow cytometry from those expressing other fluorescent proteins with shorter wavelengths, such as green fluorescent protein and Kusabira-Orange. These results suggested that the cells, tissues, and organs of pigs expressing Plum could be a useful tool in biomedical research.

Rejuvenation research provides the latest information on the molecular and cellular mechanisms necessary for most effective therapeutic approaches for human welfare. Collagen is known to be one of the elements necessary for rejuvenation. Chung et al. investigated whether treatment with collagen complexes had beneficial effects on the rejuvenation or reprogramming of adult mouse-derived fibroblasts (Chang et al. Collagen complexes increase the efficiency of iPS cells generated using fibroblasts from adult mice. pp. 145–153). They found that adult-derived fibroblasts cultured with collagen complexes showed a more youthful state, expanded at a higher rate, and exhibited reduced spontaneous cell death than those cultured without collagen complexes. Further, the efficiency of reprogramming of fibroblasts to become induced pluripotent stem (iPS) cells was significantly higher in adult-derived fibroblasts cultured with collagen complexes than in adult-derived fibroblasts cultured alone. As shown in the cover photo, these iPS cells derived from fibroblasts cultured with collagen complexes showed positive staining for stemness markers such as c-myc, Nanog, Oct4, Sox3, and SSEA-1.

It is important to identify which pluripotent stem cells (PSCs) are suitable for human medical application; however, it is unclear how the qualitative differences between different types of PSCs should be evaluated. To provide a human ES/iPS cell model, Honsho et al. converted rabbit ES/iPS cells into a naïve-like state and differentiated them in vitro into a neural lineage cells—oligodendrocytes (Honsho et al. Naïve-like conversion enhances the difference in innate in vitro differentiation capacity between rabbit ES cells and iPS cells, pp. 13–19). It is thought that naïve PSCs have much better differentiating ability than primed PSCs. Naïve-like converted ESCs differentiated much more effectively than primed-state ESCs and naïve-like iPSCs. Also, this study demonstrated that naïve-like conversion can increase the slight qualitative differences of primed-state PSCs and can be used as a valuable strategy for examining the innate capacity of human PSCs for therapeutic use.
